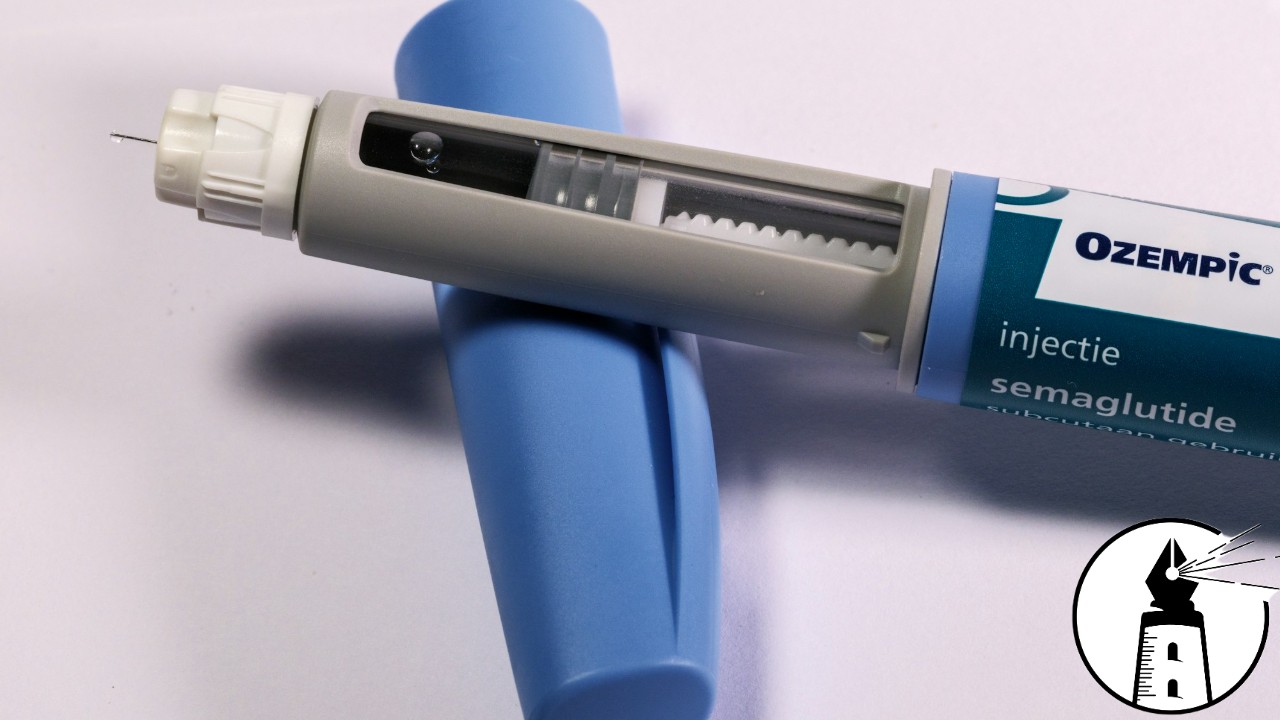
A new warning from the Food and Drug Administration (FDA) underscores growing concerns about counterfeit medications entering the U.S. drug supply. This time, the focus is on Ozempic, a popular diabetes treatment that has increasingly been used outside its original purpose. The agency recently seized counterfeit units of the drug and is now urging vigilance from patients, healthcare providers, pharmacies, and wholesalers.
The alert follows the discovery that several hundred units of Ozempic were distributed outside of authorized channels. Though the product’s lot number was valid, the accompanying serial number was not, identifying it as counterfeit. While there have been a handful of adverse event reports associated with this specific lot number, none have so far been linked directly to the seized fake drugs. Still, testing is ongoing to determine the exact nature and safety of the counterfeit samples.
At the heart of this problem is more than a simple case of fraud. Ozempic was originally developed to treat type 2 diabetes, but its effectiveness in promoting weight loss has led to a surge in off-label use. This surge has created a powerful secondary market, drawing in not only consumers eager for quick results, but also opportunistic counterfeiters looking to profit from limited supply and high demand.
As Ozempic becomes increasingly popular beyond its intended medical purpose, legitimate access becomes strained. That scarcity paves the way for unregulated sellers and fake versions of the drug to circulate. Counterfeit medications can contain incorrect ingredients, wrong dosages, or even harmful substances. They are not subject to the rigorous testing and quality controls that legitimate pharmaceuticals undergo.
While the FDA continues to investigate and test the seized products, the situation highlights a larger issue with how medications intended for chronic disease management are being co-opted for cosmetic or weight-related goals. That misuse, while sometimes portrayed as a personal choice, can ripple across the system. It affects supply chains, compromises patient safety, and ultimately creates conditions where counterfeit products can thrive.
The FDA’s warning is not only a call for scrutiny. It also reveals the unintended consequences of demand driven by trends and hype rather than medical necessity. As long as drugs like Ozempic are sought after for reasons unrelated to their approved uses, the risk of counterfeit products slipping into the system will remain a serious public health concern.
—By Greg Collier